Industry
HIGHLIGHTS
- Today's regulatory environment is changing rapidly, underscoring the need for close collaboration between safety and regulatory functions and proactive project planning. This will help strike the right balance between process transformation and regulation.
- Safety and regulatory business functions collaterally shift throughout a drug lifecycle, irrespective of scope. Enablement of safety and regulatory business functions will lead to more outsourcing opportunities and reduce operations skepticism.
- Biopharma companies aim to balance patient safety, foster innovation, and weigh the consequences of innovation.
- Adopting the right technologies aids automation, improves process effectiveness, and streamlines approaches to meet the needs of patients and industry players today.
On this page
The current regulatory scenario
The role of regulatory authorities has been growing in both the pharmaceutical industry and healthcare industries, with the former witnessing a bit more stringency.
The regulatory safety frameworks governing product and patient safety are challenging and continually developing due to the growing global and local regulations.
Pharmaceutical regulations aim to maintain and promote the manufacturing and availability of safe medicines. Biopharma regulatory and safety functions work closely together to plan and strategize the product life cycle compared to other extended functions. These two functions complement each other equally.
Biopharmaceuticals feel pressured to address current R&D challenges including a complex regulatory environment and growing regulatory expectations from marketing authorization, financial, product development, and regional pressures. Biopharmaceuticals must optimize their resources (people and processes) and technology to overcome these challenges.
On the one hand, these companies are looking for potential strategies and innovative ways to shrink timelines to introduce new products into the market—an obvious place to invest and strengthen their business vision of patient safety through innovative and orphan disease portfolios. On the other hand, they cannot neglect established products when repurposing or co-developing for newer indications. This reduces the R&D cost and establishes the products’ risk-benefit balance, which can drive revenue maximization through stronger product pipelines and optimize costs.
These underlying forces demand new operating models to manage products with cost-effective approaches, thus ensuring better patient safety and regulatory compliance.
Current operating models are traditionally managed as separate functions such as safety, regulatory, benefit-risk, medical writing, clinical data management, and biostatistics, among others. Each function designs its own business process, whereas business processes should be cross-functional to operate at best. The traditional approaches may not be as effective or the right choice for the future due to inefficiency, manual process, multiple duplicate handoffs and siloed tools and systems, and use of various vendors, resulting in gaps and redundancies. Also, these separate processes and technologies require extensive oversight and relationship management between corporate headquarters and local operating companies.
With new mandatory standards such as the identification of medicinal products (IDMP), there is a growing need to share information between these lines of businesses and integrate regulatory and safety operational expertise.
A model that caters to both aspects – regulatory and safety
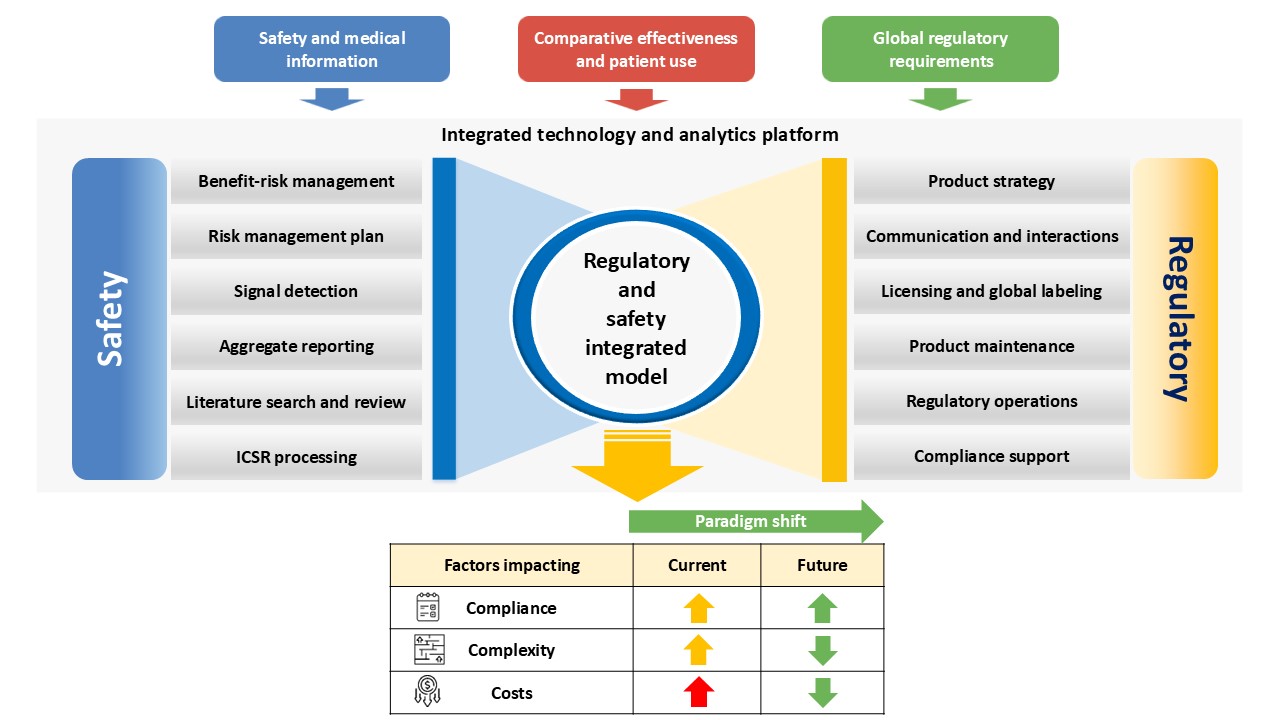
An integrated model will drive possible collaboration between the regulatory and safety functions to maximize product safety knowledge, leverage synergies, and shorten regulators’ cycle time.
Consolidating regulatory and safety functions in an integrated, transformative approach promises improved operations and compliance, reduced data discrepancies, decreased duplications, and enhanced agility in complex processes, resulting in product longevity in the market. The next generation unified technology platforms and data lakes are a part of this vision.
An interface between regulatory and safety functions presents the targeted output for regulatory submission and regulatory labeling, better product life cycle maintenance, benefit-risk optimization, annual safety reports, efficient marketing approvals, improved risk management and minimization measures, and reduced time to market.
With a futuristic, global, centralized infrastructure and workforce management under one regulatory and safety landscape, this transformational approach can benefit innovative and marketed products. Most procedures, SOPs, and changing regulations in the industry show the governance matrix between safety, regulatory, and clinical members.
Figure 1 depicts the benefits of the regulatory and safety integrated model.
Few roles of both functions can perform their duties in an integrated manner—for example, label updates are mostly safety-related but can be integrated because they are driven by labeling teams.
A competent service provider with a global presence across different regions, fluency in multiple languages, understanding of regional regulations, and experience across a broad range of therapeutic areas can drive integration effectively.
Safety and regulatory functions manage the product life cycle, while clinical is involved primarily until product development, launch, and post-market surveillance. Further, R&D departments of biopharma companies have several internal boards, panels, and committees to discuss a drug product’s safety issue, submission, new indication development, marketing approval in a particular country, and the like.
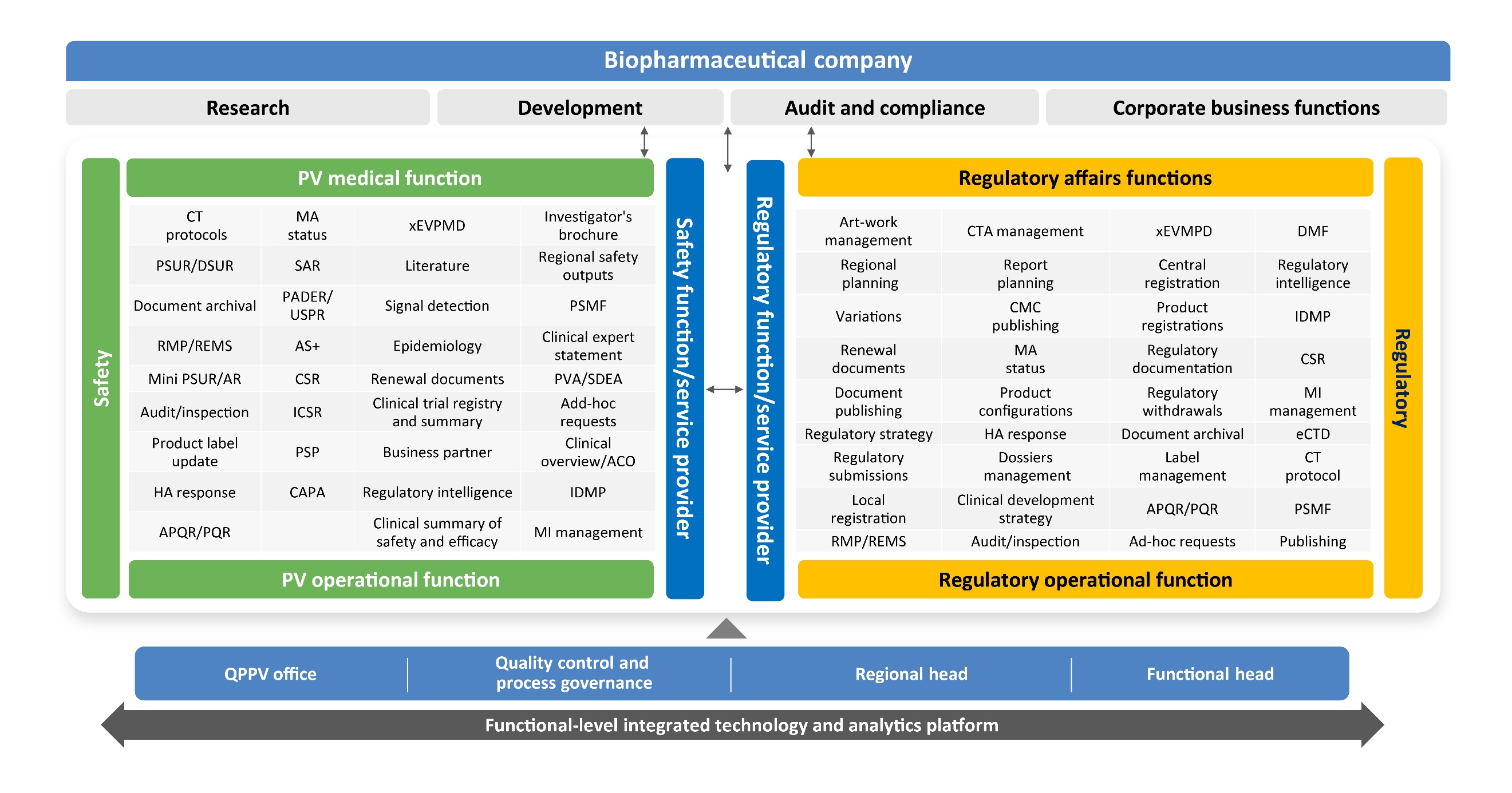
An indicative stakeholder map across both functions responsible for integration and transformation, with reduced handoffs for smoother operations, is demonstrated in Figure 2.
In our experience, such an integration has demonstrated remarkable synergies and efficiencies in day-to-day operations.
Transformation can be achieved by a talented workforce, refined training methodologies, technology, and innovation ecosystem, dedicated HR team, domain subject matter experts, black belts, and timely knowledge sharing.
The integrated model we propose also addresses the challenges of growing volumes, as deliverables can be managed more effectively. Data security measures are a must to meet product safety requirements, in addition to existing skills and knowledge reservoirs of pharmaceutical functions.
A broader vision
A robust innovative technology and transformation vision for safety and regulation is intended to provide a cost-effective solution.
The framework envisions and encourages business functions (sponsors, partners, and suppliers) to proactively foresee and manage risks.
Today, clinical e-CRF is well integrated with the safety database. Similarly, regulatory and safety documents or repositories can be integrated. Technology solutions should be designed to map key regulatory and safety processes and handshakes between these functional teams, baseline the project plans, and track deliverables compliance and variations against actual timelines.
The solutions should entail operational challenges, and present customized real-time dashboards enabling delivery excellence and enhanced compliance.
A few next-generation cognitive platforms encompass collaborative modules and provide real-time end-to-end visibility to pharma stakeholders, addressing their apprehensions during this change management. Implementation of new platforms or upgrades to existing platforms should factor in the emerging regulatory requirements.
Biopharma companies have faced challenges with disjointed systems, no end-to-end visibility or tracking, manual procedures, and complex approval processes, which have prolonged turnaround times. The new virtual ways of working have aided better collaboration across functions.
The rising need to collaborate
The demand for high-quality services and integration of regulatory safety functions is rising worldwide due to evolving regulations and a greater focus on patient safety.
Collaborative operating models enable standardization by their lucidity, harmonized decision-making, and stakeholders’ adaptation to the right collaboration tools. The key benefits of such an approach are:
- Effective regulatory intelligence: Harmonized robust regulatory understanding, process, and system impact analysis results in increased knowledge and skilled workforce.
- Skilled delivery and workforce: Experienced and well-trained resources contribute effectively to both regulatory and safety activities and meaningfully engage with pharma stakeholders and vendors.
- One-stop solution: A single service provider for end-to-end regulatory and safety deliverables instills a stronger sense of ownership and presents opportunities for innovation.
- Collaborative, tech-enabled dashboards: A single, integrated view of enhanced metrics and analytics offers in-depth visibility toward risk identification, management, and mitigation of compliance risks providing transparency to the customer, reducing oversight.
- Global and local presence: This approach assures fully compliant deliverables as resources possess knowledge of local and regional regulatory requirements.
- Increased operational agility: Adoption of a systematic and an agile approach ensures process approaches ensures a synergistic relationship between regulatory and safety functions and cost-effective operations, and improved effectiveness during the transformation phase. Along with drug safety approvals, it accelerates the implementation of automation leading to enhanced quality deliverables.
We believe that the above approaches can generate more opportunities and reduce operational scepticism related to business functionality.