Industry
HIGHLIGHTS
- Efficient and effective pharma labeling and artwork management is critical for better health outcomes and patient safety. Drug supplies are dependent on better label management, which needs to be compliant, correct, and consistent.
- There is a need for reimagination of label and artwork management to reduce the overall cycle time of new label creation, faster regulatory approval, and change implementation towards achieving on-time supplies and faster time to market.
- A technology-enabled, integrated approach to packaging, artwork, and labeling management can redefine the whole process and provide multiple benefits.
On this page
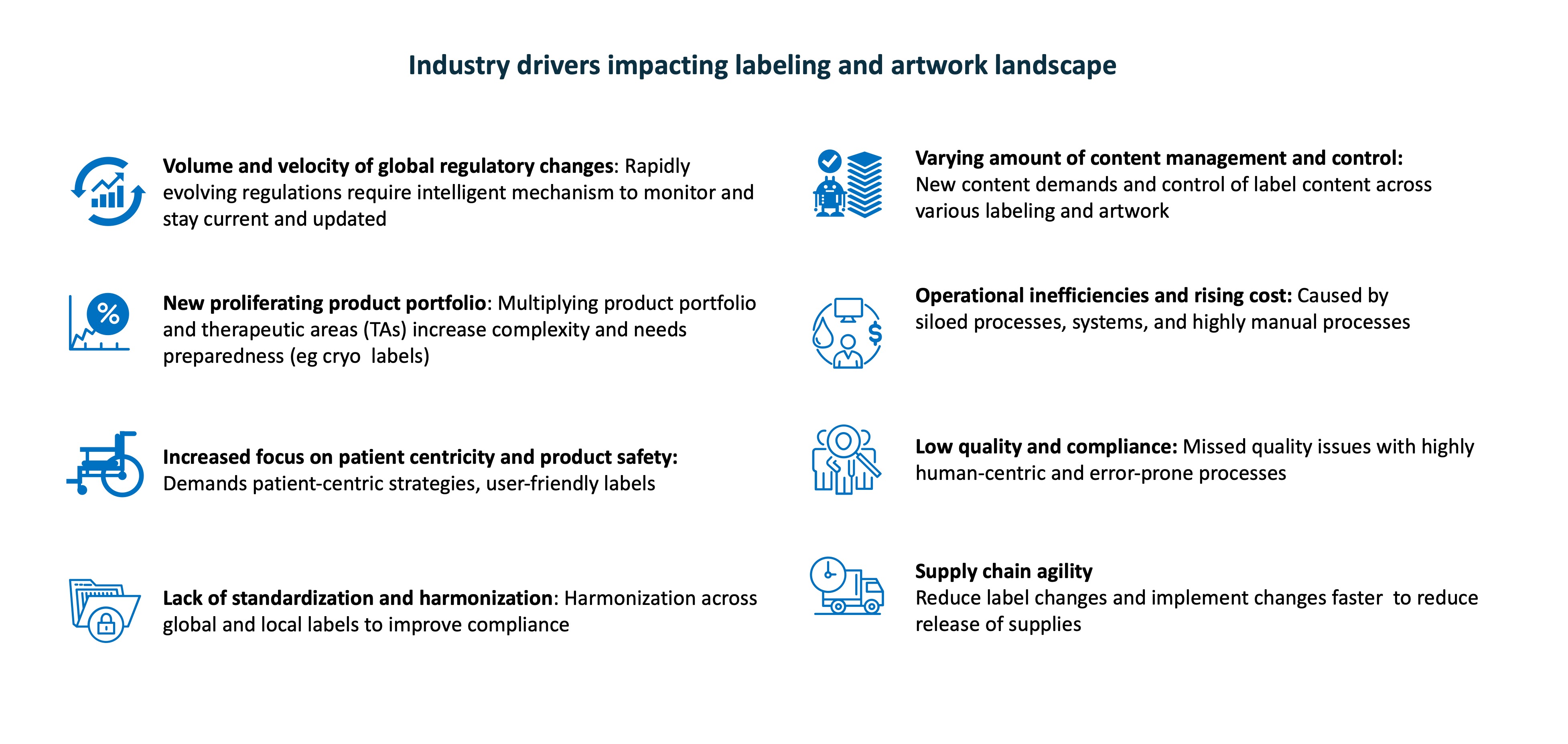
Industry drivers
The complexity of the labeling landscape is well understood by considering one global drug with multiple formulations, dosages, packaging, languages for localization and format versions.
Any change in the product label data must be updated in all related documents and tracked for compliance and consistency across geographies. Additional content for geo-specific localization also needs to be managed and tracked. Industry drivers that demand reimagining labeling management include rapidly evolving regulatory landscape, proliferating product portfolios, new and varying demands on content management and controls, operational inefficiencies, and rising costs due to organizational silos. A lack of standardization and harmonization has a cascading effect on quality and compliance. More importantly, patient-centricity, patient safety, and supply chain agility required to accelerate drug launches are driving the need for a fresh approach to labeling.
The reality
Reimagining the current state of label and artwork management is essential to accelerate new label creation, approval, and change implementation for on-time supplies and faster product launches.
Accurate and consistent labeling can improve patient safety and quality compliance, resulting in reduced product recalls and fewer changes eventually reducing cost burden. To achieve this objective, industry approaches like shift from document to data-centric, non-standardized to standardized, and sequential to parallel submissions can facilitate transformation in pharma labeling and artwork management.
Integration is key
In the digital maturity curve, labeling and artwork management needs to be considered as highly automated and intelligent.
This can be achieved through an integrated discipline called Integrated Packaging, Artwork, and Labeling Management. This gives a framework for pharma companies to re-think their packaging, artwork, and labeling processes by leveraging a unified platform as a single source of truth.
Integration of siloed systems supplemented with product master data enables efficient production, approval, and management of packaging, artwork and labeling with seamless information flow, streamlined regulatory compliance, effective collaboration, and automated workflows. Label requirements are increasing due to patient-centricity, new drug types, and mergers and acquisitions. Scalable, accessible, personalized needs can be met through a cloud-based integrated approach that calls for seamless deployment, upgrades, and maintenance.
The integrated ecosystem allows the flow of information within a single platform effectively addressing knowledge management needs for knowledge-driven intelligence and insights. A change control system can register the label change request. Event capture, tracking, and publishing can be managed with a Regulatory Information Management (RIM) system. A centralized content management system with reusable content blocks and templates can support integrated labeling, artwork, and packaging systems.
The entire ecosystem enables seamless information flow through both upstream label change request to content creation for submission and downstream artwork production. This will pave the way for a highly efficient and effective labeling and artwork system with significantly less manual burden and improved compliance.
The tech edge
An integrated packaging, artwork, labeling management approach enabled by highly automated and intelligent capabilities can bring transformational benefits.
By leveraging cutting-edge technologies, the capabilities will offer the following advantages:
Staying up to date with regulatory changes and enabling faster change implementation
Missing new regulatory requirements can expose companies to multiple risks like non-compliance, delayed submissions, and supplies. Given the proliferation of information sources, manual capturing of regulatory revisions, their analysis, and information dissemination has become challenging. Digital technologies (AI/GenAI, knowledge Graphs) can sense regulatory changes autonomously from relevant sources (e.g., guidance), extract, store, index the information, and, based on rules, classify them as likely to have high, medium, or minimal impact, and notify relevant stakeholders. Intelligent integrated labeling processes driven by AI-ML, business process management (BPM) technologies can bring improved efficiency through less human intervention and enable faster supply release.
Processing high volume of regulatory information
Mining of regulatory guidance and extraction of obligations is highly manual and time-consuming. Technology like AI, GenAI can enable structured extraction of obligations from long, unstructured guidance documents and classify and summarize the information for experts to further curate and justify the proposed change. The curated, summarized obligations can serve as an input for labeling strategy documents.
Impact assessment of regulatory label change
Finding the relevant impact of the change is highly human-centric and dependent on tacit knowledge. Knowledge-driven trained models, rule-based decision-making, and intelligent querying can enable impact assessment using a structural knowledge repository, and cognitive and analytical capabilities to find patterns and relations and provide views to support the right decision-making.
Content generation for new or updated labels
The manual and redundant activity of content creation and reuse is time-consuming, error-prone, and adds no value. The packaging, artwork, and labeling processes can leverage standardized content from a structured content repository. Any single change in the content can reflect in other mapped documents, thereby, preserving the integrity of the information in the label about drug composition, manufacturing details, expiry date, and consumption instructions.
Technologies like knowledge graphs, AI, and GenAI can help content creation by defining new document structure, edit and store the standardized content in structured graphs that link, relate, and analyze data based on the context. For e.g. company core data sheet (CCDS), summary of product characteristics(SmPC), United States Prescribing Information(USPI), and patient information leaflet(PIL) leverage the same data.
Manual label quality review
Frequent label and artwork changes make content quality review a time-consuming and resource- and effort-intensive task. Quality review by using robotic process automation (RPA), BPM, AI, or GenAI can enable consistency, improve quality, traceability, and visibility, and decrease latency through better workflows and by eliminating manual tasks.
Discrepancy management and harmonization
Ensuring consistency and compliance of labeling documents globally is highly manual, error-prone, and time-consuming. AI, GenAI can check deviations across various SmPCs (country-based) by comparing and providing a report on differences. It can also enable harmonization across SmPCs by using prompts and digital assistants, reducing the manual load significantly.
Localization and translation of labeling
The right translation of labeling and artwork is vital for regional and local marketing of a product. The need for high-quality translation for a wide range of stakeholders, covering country specifics, cultural contexts, medical terminology, and adherence to local regulatory requirements are challenging. AI, GenAI can enable significantly faster translation of SmPCs into local languages and back to English. Medical ontologies and internal and tacit knowledge-driven large language models (LLMs) can produce high-quality first drafts of the translated label that can be sent for review. It can potentially reduce the addressable spending on translation services and enable faster label submissions.
HA query response management
Generating responses for similar queries from multiple authorities on label artwork is highly time and effort-driven and dependent on tacit knowledge. By leveraging a historical repository of indexed HA (health authority) query and responses, AI, GenAI can enable auto response generation for similar queries, saving time and effort. It can also provide precedence intelligence on propensity and type of queries across the globe, improving new submissions.
Mock artwork designing
Generation of mock artwork designs, their review and approval are highly manual and redundant (given that they are mock). Auto-population of mock artwork templates using metadata-driven digital tools can significantly reduce time and effort and improve quality.
Competitive intelligence and due diligence
Labeling and artwork creation requires intelligence on competitors to build and refine strategies. AI, GenAI tools can help gather this intelligence and use it for differentiation and compliance checking. It can improve compliance and help with high rates of approval.
Integrated technologies have immense potential to transform labeling and artwork. To realize the value of the unified vision, supplemented with centralized knowledgebase, in-depth analysis of processes and maturity road map of technology needs to be further explored.