Industry
HIGHLIGHTS
- In 2017, the Medical Device Regulation (2017/745/EU) (MDR) and In-Vitro Diagnostic Medical Device Regulation (2017/746/EU) (IVDR) arrived at a directive regarding the production and distribution of medical devices across Europe.
- The new regulatory requirements had to be adhered to within four years.
- The EU MDR deadline has been extended by another few years, giving medtech manufacturers some leeway to align with the new requirements and prepare for the future.
On this page
Future readiness
The European Union Medical Device Regulation (EU MDR) has introduced a new set of regulatory requirements.
These requirements govern the production and distribution of medical devices in Europe for technical advancement and enhanced traceability of medical devices.
The same also applies to imported devices manufactured elsewhere but sold in Europe. Last year, the regulatory body extended the timeline for this transition from the earlier directive to ensure adequate availability of medical devices. This extended timeline provides manufacturers greater flexibility to assess their present manufacturing processes and systems for future readiness.
In 2017, the Medical Device Regulation (2017/745/EU) (MDR) and In-Vitro Diagnostic Medical Device Regulation (2017/746/EU) (IVDR) brought EU legislation on the same page for technical advancement, better traceability, and overall progress in medical device law making across European countries. The new standard will replace the existing medical devices conformity directive to Directive 93/42/EEC of MDD.
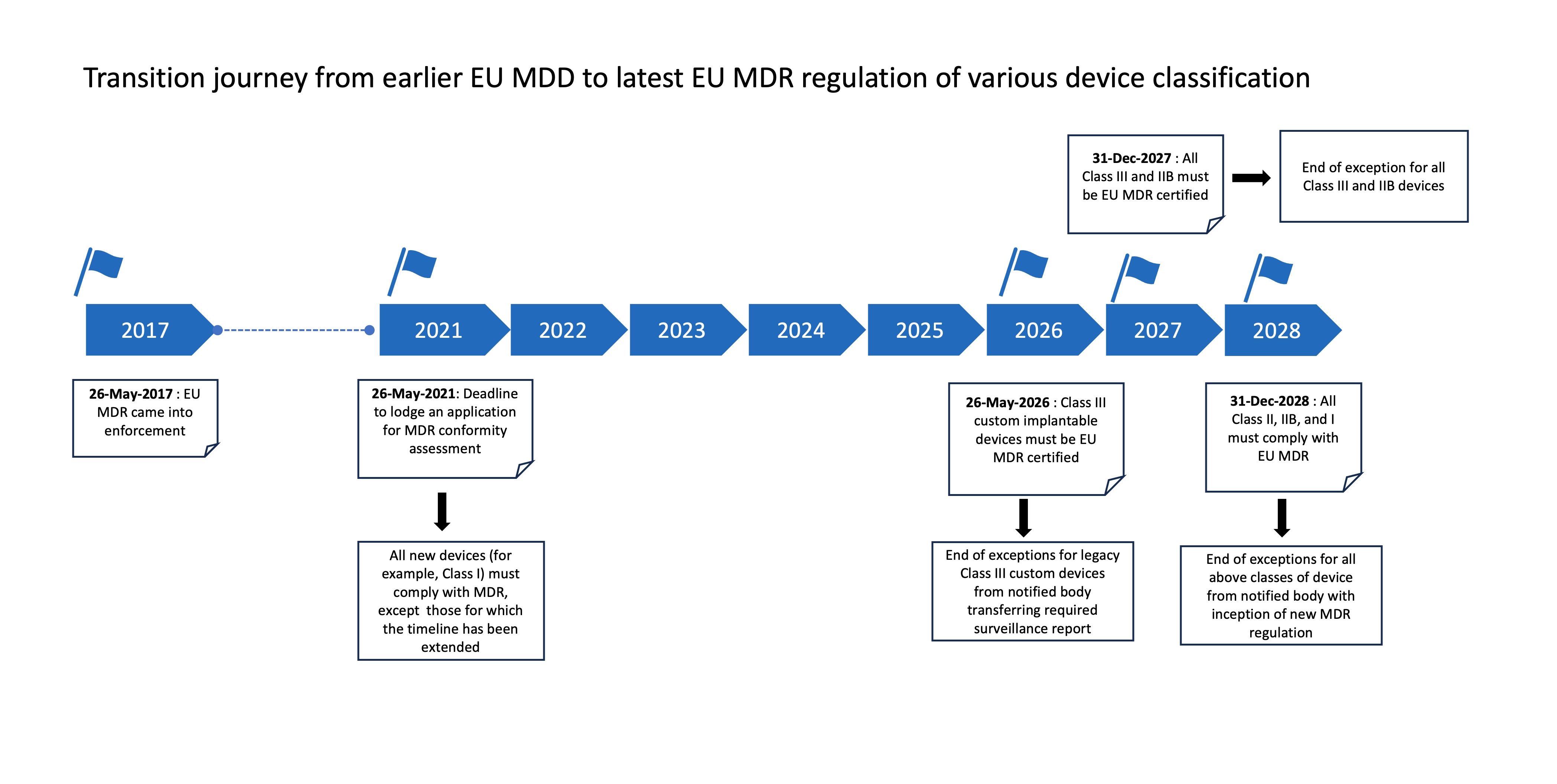
The roadmap
In 2017, when MDR was published, it had aimed at a four-year transition period.
However, to address high-level public health protection and significantly avoid any shortages of medical devices, both manufacturers and notified bodies have now agreed on sufficient time for this transition. Moreover, the dismissal of the sell-off date will prevent the unnecessary disposal of safe devices. The EUMDR adoption will be in gradual phases and will take over four years.
Medtech customers are already aligning their current processes and systems per the new regulation, so the extended transition period will provide headroom toward building an adaptive, resilient, and future-ready smart factory.
The starting line
Some of the manufacturing operation functions within the value chain seek urgent attention.
The functions that need to be observed include the following:
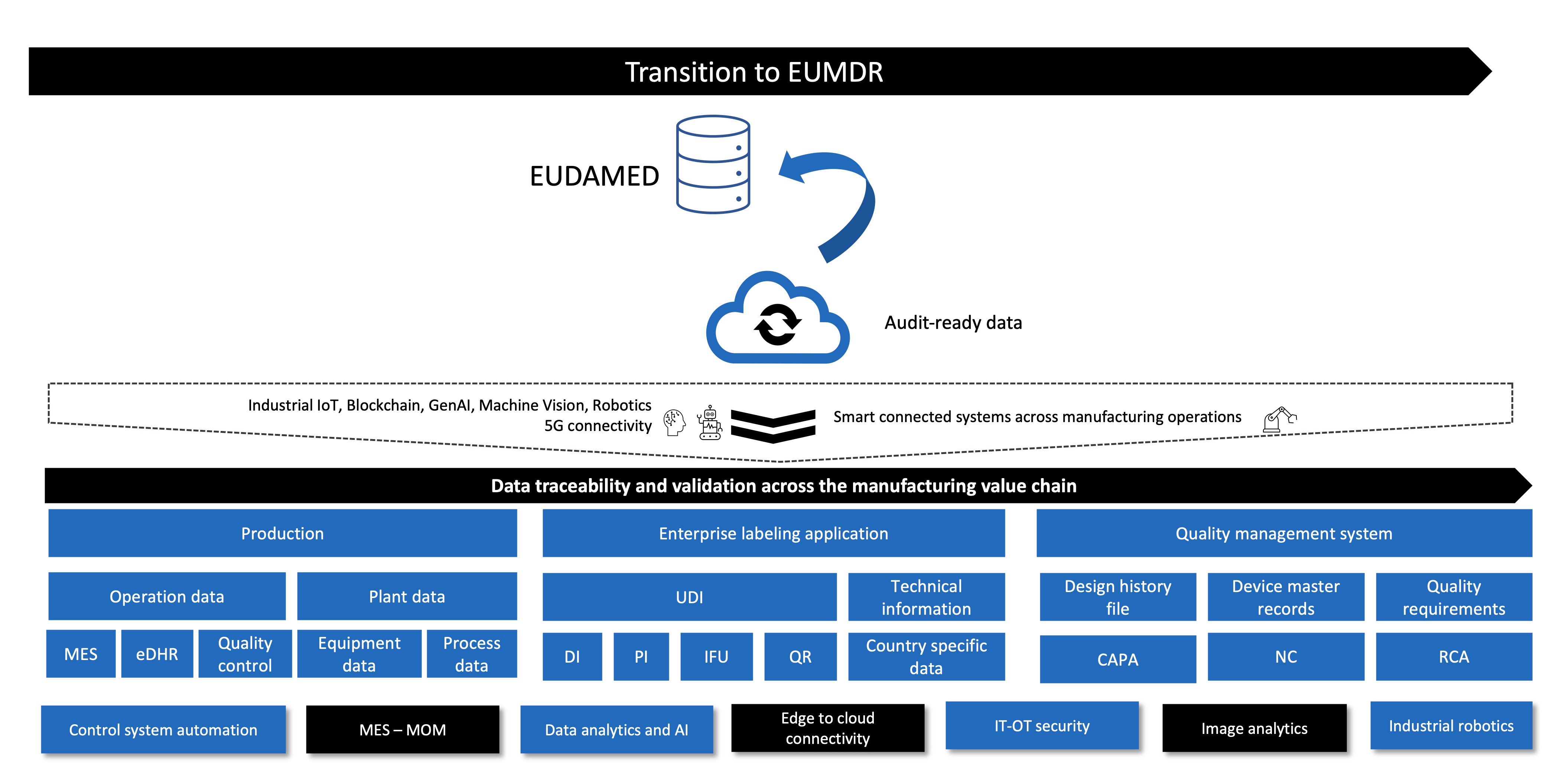
- Design to manufacturing – Right from innovation to product development, some of the most critical elements of traceability are design and documentation. A digital thread solution compiles all the documents into a single source of truth to enhance product lifecycle control. It digitizes the development information, adopts model-based engineering practices, and enables seamless integration with manufacturing and component sourcing processes. A well-managed and connected bill of materials (BOM – eBOM, mBOM, sBOM) is essential for overall traceability across engineering, manufacturing, and service value chains. This makes them adaptive for faster change execution for improved product quality with timely reporting.
- Manufacturing operations – Manufacturers need to ensure strict quality control across the value chain while closely tracking the device through the unique device identifier (UDI) and device history record (DHR). Paper-based SOPs, work instructions, or DHRs are no longer viable for recording and timely reporting in EU MDR’s centralized database, the European Database of Medical Devices (EUDAMED). Even though the legacy MES/MOM offers various feature-rich modules that integrate production data across disparate systems, it demands a lot of effort, time, and investment for enterprise rollouts. So, manufacturers need to decide the right approach toward paperless manufacturing, a complete MES/MOM solution, or cloud-based no-code cloud operation platform that will digitize production workflows into an electronic form of DHR (eDHR).
- Product labeling – Every device will have a product label with a UDI, which contains a static portion as a device identifier and a dynamic production identifier (PI). The device identifier (DI) contains fixed device codes, while the production identifier (PI) contains device serial numbers, lot numbers, and expiration dates. The QR code will provide necessary information intended for patients, instructions for use (IFU), and other technical components. And a cloud-based agile label management application will essentially centralize the production data from various systems (MES and ERPs), expedite the label change management, enable data-driven label control, and ensure full auditing with reporting capabilities. The QR code enables further visual interaction with patients and HCPs, leveraging augmented reality (AR).
- Quality control – The European market caters to 27 member states, with other countries falling under the European economic zone. The devices need to be suitable across diverse geographies and populations. Hence, a smart quality control process that leverages image analytics and AR-VR technology to build a robust, data-driven validation mechanism for immediate corrective action in case of adverse events is essential.
- IT-OT security – Industrial control system automation layers were isolated and inaccessible to cyber attackers. However, with Industry 4.0 and decentralized operations, the convergence of OT and traditional IT layers is making shop floor devices and systems more susceptible to cyberattacks. A secure IT-OT framework will safeguard the plant systems from unauthorized access or unplanned downtime and secure your journey toward the factory of the future.
Assessing the present state
Manufacturers must assess the present state and build on connected, collaborative, and cognitive value chains across the production life cycle.
Manufacturers needs to assess the present state and start building on connected, collaborative and cognitive value chains across the production life cycle utilizing given transitions period by EUMDR and move toward paperless manufacturing and shift the gear from person dependent to data driven decision making and unlock the true potential of next gen technologies artificial intelligence (AI), machine learning (ML), image analytics, and generative artificial intelligence (GenAI) from design development to product testing.
A future-ready smart factory framework will build a solid foundation to embrace regulatory needs and focus on addressing market needs with innovative solutions.